The European Union (EU) has established medical device regulations to protect the public from potentially unsafe or ineffective products. As part of this framework, manufacturers of high-risk medical devices must prepare a Periodic Safety Update Report (PSUR) for each device in their portfolio. This article will discuss the purpose of PSURs and the detailed requirements for their creation, as outlined in Regulations (EU) 2017/745 and 2017/746.
The primary purpose of a PSUR is to collect information on the safety and performance of medical devices over time. The report is required whenever there have been significant changes to a device or if its safety or performance has been questioned. By regularly monitoring device performance, identified risks can be quickly identified and addressed. For example, a PSUR will alert authorities to malfunctioning parts or features and enable the development of preventive or corrective actions to reduce associated risks.
The PSUR is an important document that provides a comprehensive overview of the safety and efficacy of drugs used to treat a disease or medical condition. PSURs help ensure patients' safety and provide valuable insights into the long-term effectiveness of the drugs.
To understand the PSUR requirements properly, the European Commission created the MDCG 2022-2021. A work program for implementing the Medical Devices Regulation (MDR) by the European Commission. It sets out the tasks that the European Commission, competent national authorities, and other interested parties should carry out to ensure a successful transition to the new regulatory environment.
PSURs for MDR Certified based on their Classification:
Flow diagram for Medical Devices certified under EU 2017/745 based on the classification (Including Custom Made Devices [CMD])
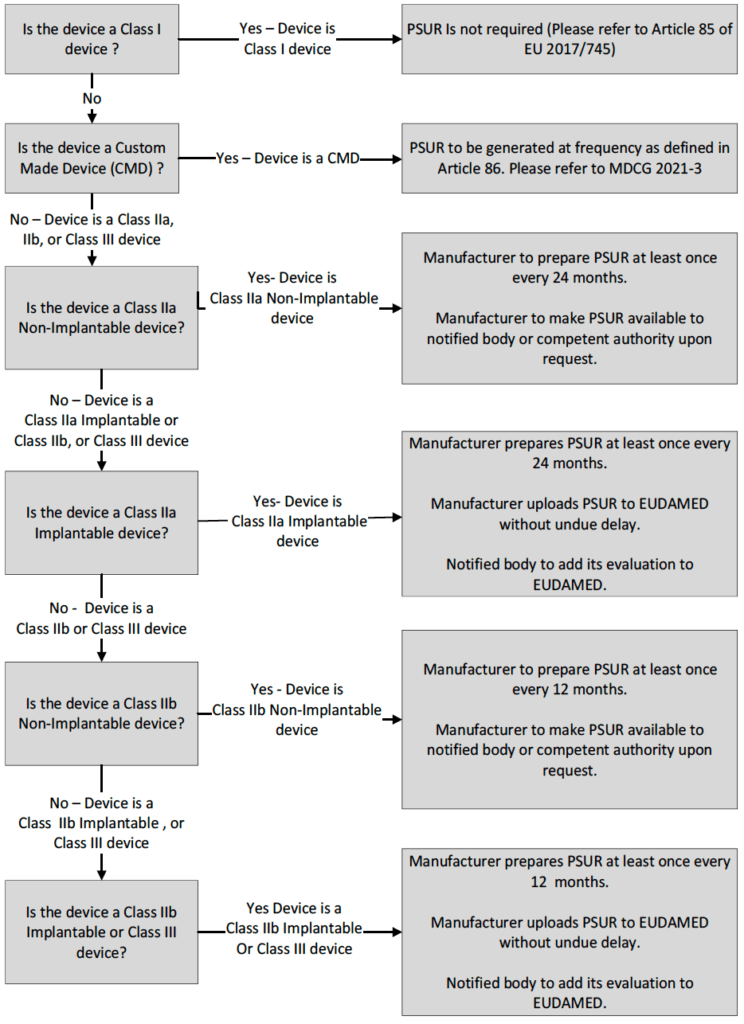
The primary objective of PSURs is to assess the risks and benefits of the medical device being reviewed. This means evaluating all available safety data – including clinical trial data, Post-Marketing Surveillance (PMS) data, and adverse event reports – to analyze the risk-to-benefit ratio of the medical device. In addition to assessing the potential side effects, a PSUR may also include a review of its efficacy and cost-effectiveness.
The other objectives of a PSUR are to identify necessary new safety signals, evaluate changes in the risk-benefit profile, and assess the impact of any proposed changes to the labeling and warnings. This evaluation of the objectives is a critical step in ensuring that the medical device remains safe and effective for its intended use. The goals of the PSUR include a comprehensive review of the product, its performance data, complaints, and risks associated with the device.
In evaluating the objectives of the PSUR, it is essential to consider the device safety evaluation of laboratory tests, consumer feedback, and any potential risks that may lead to consumer injury or death. The evaluation should also evaluate the product's effectiveness in its actual use. Additionally, the evaluation should consider any potential safety issues associated with the product’s design.
The evaluation should also include an assessment of the product’s compliance with regulatory requirements, including labeling, warnings, and ingredients. This assessment should consider public health, safety, and the environment.
The Periodic Safety Update Report (PSUR) shall include the following information:
Also, you can find a sample template for the PSUR in Annex I of the MDCG 2022-21; templates for the presentation of data in the PSUR in Annex II; general Information related to the presentation and assessment of the collected data by the manufacturer in Annex III; PSUR requirements – summary table for MDR and legacy devices in Annex IV; PSUR web form for the manufacturer in Annex V.
Workflow for assessment of PSUR requirement:
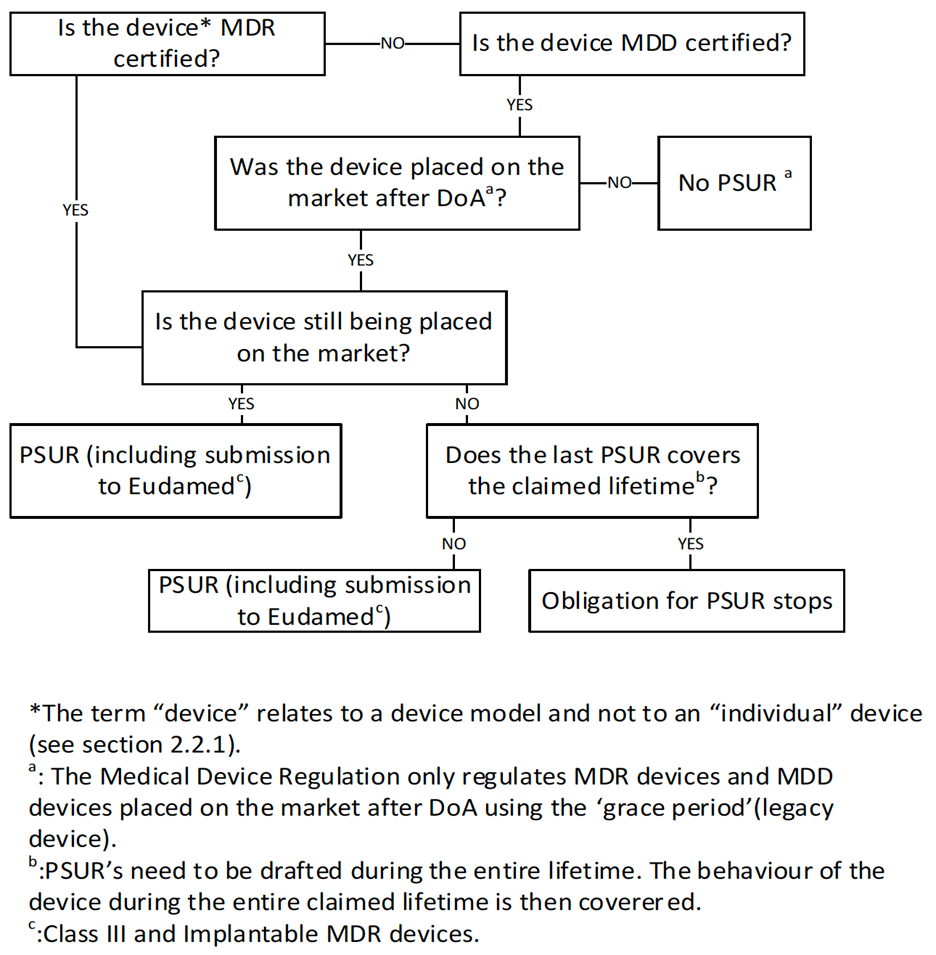
Until EUDAMED is fully operational, manufacturers or their authorized representatives must comply with national provisions and consider MDCG 2021-1 Rev.1.12 for Class III and Class IIa or IIb implantable medical devices certified under the MDR. For Notified Bodies, the manufacturer should submit their PSURs appropriately. For Competent Authorities, the manufacturer should provide their PSURs when requested.