
With the implementation of the EU Medical Device Regulation (MDR) just around the corner, the European healthcare industry is feeling a mix of emotions. On the one hand, it is exciting to finally put the oft-delayed regulations into action and reap the benefits of a restructured, more stringent, and harmonized medical device regulatory framework. On the other hand, there is also a sense of frustration and confusion as the industry scrambles to comply with the many complicated requirements before the deadline

One primary source of this confusion is the delay in the full implementation of the MDR. In June 2020, the European Commission announced a one-year delay due to the disruption caused by the COVID-19 outbreak. This delay changed the original timeline, resulting in a new staggered implementation schedule that extends across most of 2021. This shift has created further uncertainties for the industry as companies scramble to adjust or accelerate their plans. In addition to the confusion, the delays have made investments in new processes, methods, and systems less likely, which can mean cumbersome processes for businesses seeking to keep up with regulatory changes
The delay of the MDR has also caused further logistical issues for the industry, as companies face tight deadlines and off-target compliance with the new rules. With just a few weeks left until the delayed due date, companies are under immense pressure to make sure they are fully compliant with the MDR before it all goes into effect. For instance, companies must document all changes made during their transition to ensure their products comply with the MDR. This documentation needs to be audited and validated before the introduction of the MDR, which may not be possible in the limited time remaining for the industry to prepare
The industry must work together to provide clarity and flexibility for companies facing tight timelines to ensure a smooth transition and compliance with the MDR. For example, companies should be offered guidance and support from industry bodies to help ensure they comply with the MDR efficiently and effectively. In addition, industry bodies should also provide companies with tools and resources regarding the MDR, such as available support mechanisms, risk management processes, and quality assurance methods. The delayed MDR can be successfully implemented with minimal disruption by encouraging greater collaboration within the industry and providing companies with the necessary resources and guidance
The delay of the MDR in January 2023 is an unfortunate reality that the industry must accept. The new regulation was designed to ensure safety for medical device users and was scheduled to take effect on May 26. However, due to technical and administrative issues, the European Commission postponed the implementation
Under the proposal, the transition period deadline is extended from 26 May 2024 to 31 December 2027 or 31 December 2028, depending on the risk class of the device. High-risk devices would be subject to the shorter transition period ending in 2027, while low- and medium-risk devices would have until the end of 2028 to complete a conformity assessment
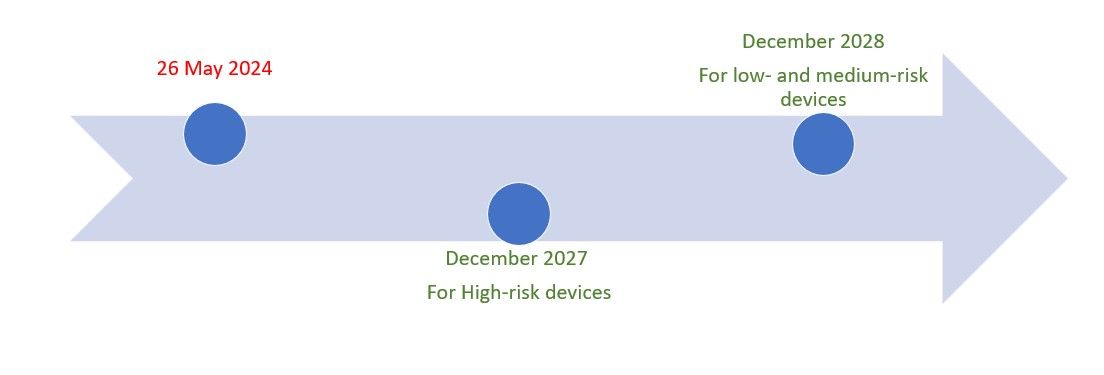
For class III custom-made implantable devices, the Commission agrees to create a new transition period until 26 May 2026 to give manufacturers more time to certify their quality management systems by a notified body. For such manufacturers, the new transition period will only apply if the firm has applied to a notified body by 26 May 2024 and has signed a contract for the certification by 26 September 2024
For instance, most manufacturers are now required to meet additional stricter standards in developing medical devices. This will likely lead to higher development costs and an estimated loss of up to 15.7 billion euros by that date. Furthermore, medical device manufacturers could suffer lost sales due to decreased purchases of existing non-compliant medical devices
The consequences of the delay also extend to consumers and patient safety. People with chronic conditions such as diabetes and cardiovascular disease rely on medical devices to manage their needs. With the delay in the new regulation, their devices may not be as safe as they would have been after the new rules took effect. Further, the uncertainty may lead to people needing more confidence in their devices
Therefore, the one-year delay in the new medical device regulation is a significant setback for the industry. It causes financial losses and delays in producing safe devices and can create consumer unease. However, the positive side is that companies will have the opportunity to ensure that their devices meet the new safety standards and that consumers will have access to better and safer devices. The industry will have to accept this unfortunate reality and adapt accordingly
The EU's announcement on the postponement of the new Medical Device Regulation's launch dates: https://health.ec.europa.eu/system/files/2023-01/mdr_proposal.pdf